A new process for the preparation of diamond by American scientists at room temperature and pressure
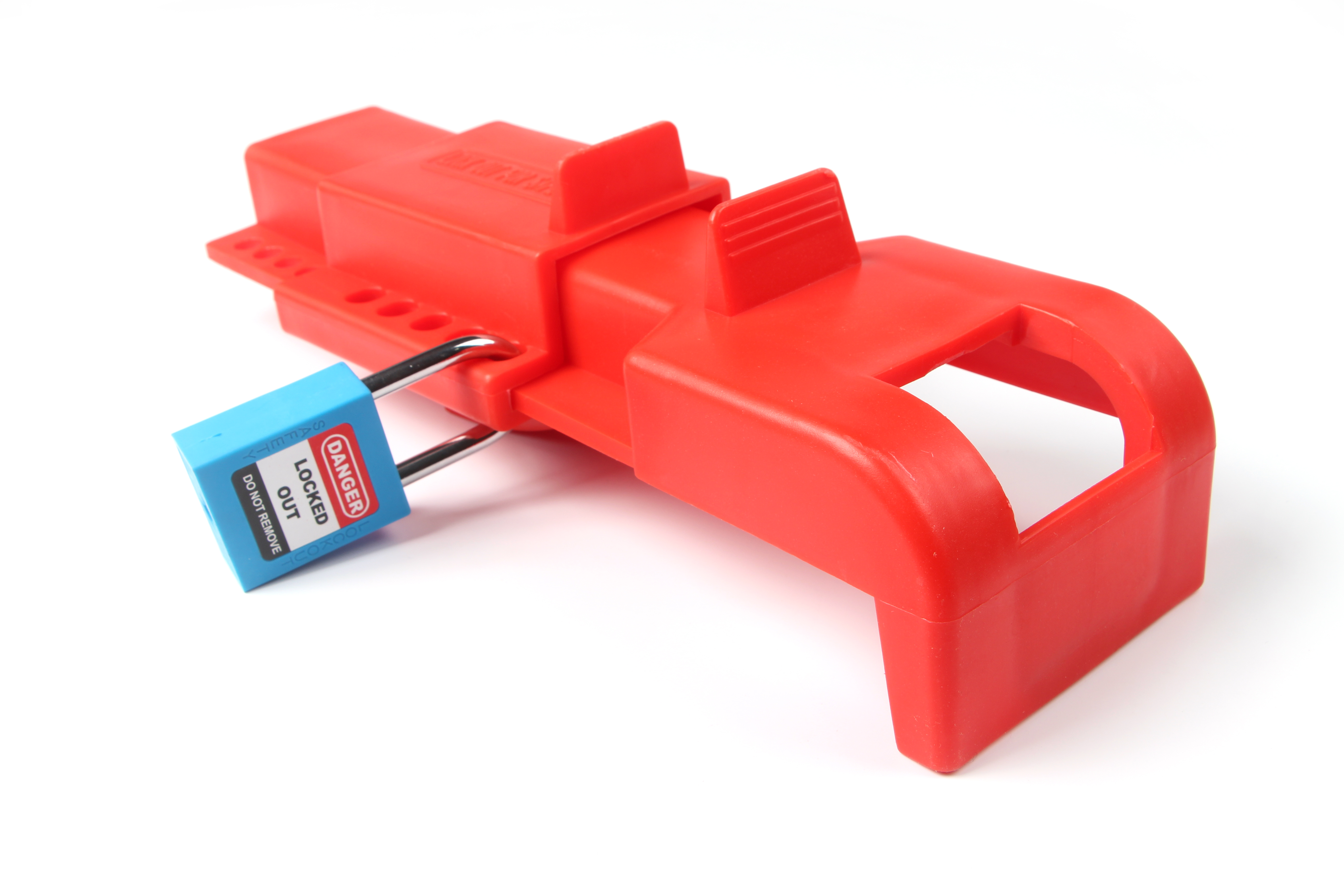
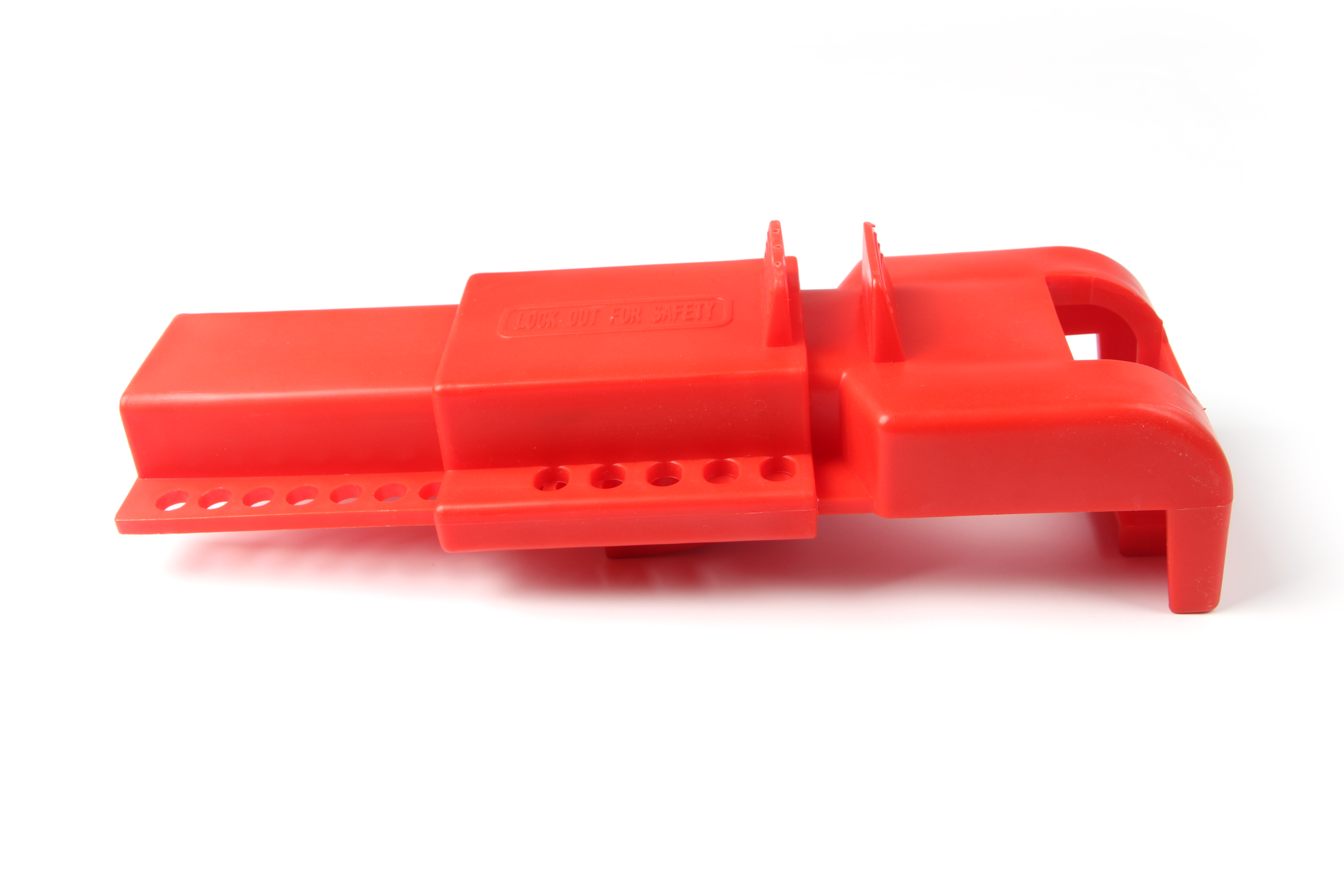
Butterfly Valve Lockout system is Easy-to-use. Designed to immobilize valve by encapsulating Butterfly valve to prevent accidental energizing. Our butterfly Valve Lockout is made from polypropylene. This valve lockout have a compact design, which is very easy for carrying and storage,.It can be used together with safety padlocks or safety lockout hasps.
Butterfly Valve Lockout Butterfly Valve Lockout,Valve Lockout,Butterfly Valve Lockout Devices,Oversized Butterfly Valve Lockout Lockey Safety Products Co., Ltd. , http://www.lotolockey.com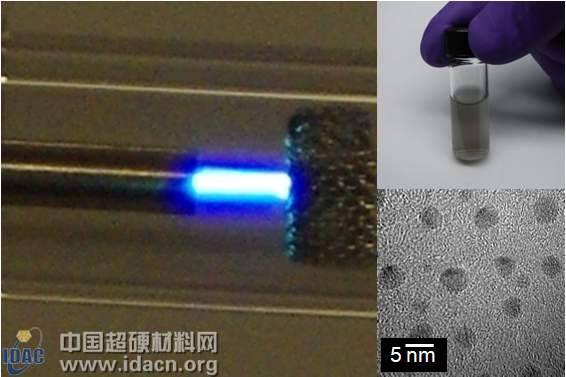
The illustration shows ethanol vapor separated by microplasma under an electron microscope; carbon particles are collected and dispersed in a solution
This technical achievement, published in the journal Nature Communications, is an innovative breakthrough in the Case Western Reserve University Diamond Research Program.
Mohan Sankaran, an associate professor in the Department of Chemical Engineering and a leader in the project, said that ethanol vapor is converted to diamond at room temperature and pressure, a process that is not complicated. The ethanol vapor is pumped into the plasma and the nanodiamond particles are finally produced by adding hydrogen. This type of diamond can be prepared by any laboratory as long as the conditions are appropriate.
Due to the normal temperature and pressure conditions of the preparation process, materials such as plastic will not be melted, which provides great convenience for the application of some high-end technologies. Diamond is known for its high hardness, superior optical properties and high thermal conductivity. Unlike other forms of carbon and graphite, diamond is a semiconductor that, like some properties of silicon, is an important material in the electronics industry.
Although the process of preparing diamond is simple, it is not too short to grasp the precise gas concentration and gas flow.
Sankaran and John Angus, retired professors at Case Western Reserve University, proposed the idea of ​​preparing nanodiamonds under normal temperature and pressure conditions eight years ago. In the 1960s and 1970s, Angus used high-temperature and low-pressure methods to prepare diamond films, which are now commonly used in chemical vapor deposition (CVD). Sankaran uses nano-diamond particles to produce micro-diamond particles.
Now, the preparation of diamond usually requires high temperature and high pressure conditions to convert graphite into diamond; or hydrogen is combined with a heated substrate to grow diamond.
“In terms of nanometer size, surface energy makes diamond more stable than graphite,†explains Sankaran. “If nucleation of 5 nm carbon clusters in the gas phase can be achieved, diamonds can be achieved at atmospheric pressure at room temperature. Preparation".
First, the researchers developed a plasma that has a gas-like nature and is partially charged or ionized. A spark is a plasma, but its temperature is high and uncontrollable.
In order to create a safe low temperature environment, the worker pumped argon gas into a pipe with only the diameter of the hair wire and ionized it to obtain a microplasma. This microplasma is shaped like a burning fuel. Then, the researchers pumped the carbon source, ethanol, into these micro-plasma, so that the carbon is not affected by other molecules in the gas, resulting in particles of 2 to 3 nanometers in size, and the final product is nanodiamond.
Immediately after less than 1 microsecond (one millionth of a second), the researchers continued to input hydrogen, which allowed them to remove carbon that had not yet become diamond particles while still maintaining the stability of the formed diamond particles. Sex.
At present, only the diamond micropowder is prepared experimentally, and the size of the jewelry-grade diamond has not yet been reached. Sankaran and Kumar have been working tirelessly to keep the average diameter of diamonds around 2 nanometers.
It took the team nearly a year to verify that the final product they produced was diamond and whether the process could be replicated. Kumar introduced that many different methods were used for verification, and Raman spectroscopy was also used to analyze the composition of these nanoparticles.
At present, nanodiamonds are usually prepared by an explosion method, and carbon phases are transformed into diamond particles under ultrahigh pressure and high temperature conditions which are instantaneously generated and disappeared; time-consuming and short-cost are low. However, diamond particles tend to cluster, and their size and purity are different. The new diamond preparation method of Case Western Reserve University has solved these problems well, and the diamond has higher purity and smaller particle size.
Three types of diamonds were prepared in the experiment: cubic, similar to diamond structure; hydrogen-containing; hexagonal. Hexagonal diamonds have been frequently discovered by scientists in outer space in recent years. According to research, the high pressure generated by the impact of interstellar dust in outer space is actually involved in the process of transforming graphite carbon into diamond in outer space, commonly known as stellar diamond. Sankaran said: "This new method of making diamonds may be the same as that of outer space stellar diamonds; in fact, plasma and ethanol are also present in outer space."
The team is further investigating whether the process can be improved to produce diamonds with specific requirements and to control their performance. Among the above three diamond types, the hexagonal diamond is higher in hardness than the cubic diamond.
Achieving large-scale production is also the next step of the research team. Sankaran is very optimistic about its prospects: In the future, as the technology matures, cheap and simple preparation processes will bring more innovative and advanced applications to the industrial sector. (Compiled from "Nanodiamond production in ambient conditions opens door for flexible electronics, implants and more")
This universal sized Butterfly Valve Lockout device has an innovative design which enables to lockout most of the butterfly valves used for various applications. This is a simple yet an effective way to secure your butterfly valves to prevent unauthorized operation.